Pharmaceutical GMP Compliance
Pharmaceutical GMP Compliance
 Biotech and Pharmaceutical to get GMP compliance, Core Compliance provides a clear road map of requirements. Good Manufacturing Practice (GMP) is a system for ensuring that products are consistently produced and controlled according to quality standards. It is designed to minimize the risks involved in any pharmaceutical production that cannot be eliminated through testing the final product.
Biotech and Pharmaceutical to get GMP compliance, Core Compliance provides a clear road map of requirements. Good Manufacturing Practice (GMP) is a system for ensuring that products are consistently produced and controlled according to quality standards. It is designed to minimize the risks involved in any pharmaceutical production that cannot be eliminated through testing the final product.

Good Laboratory Practice (GLP) for pharmaceutical and biotech companies represents a quality system of management controls for research laboratories and organizations to ensure the uniformity, consistency, reliability, reproducibility, quality, and integrity of industrial chemicals, pharmaceutical drugs/dietary supplements & Cosmetic products. Basic requirements include, design, location, equipment, chemicals & reagents, documentation, reports and auditing.
Core Compliance has FDA industry expertise in-depth knowledge of cGMP regulations from clinical development through post-approval drug and biotech product manufacture. We provide FDA consultants that subject matter expertise’s in requirements, regulations and a clear path to GMP, GLP compliance with onsite and online flexibility.
See FDA facts about current good manufacturing practices– FDA GMP Facts
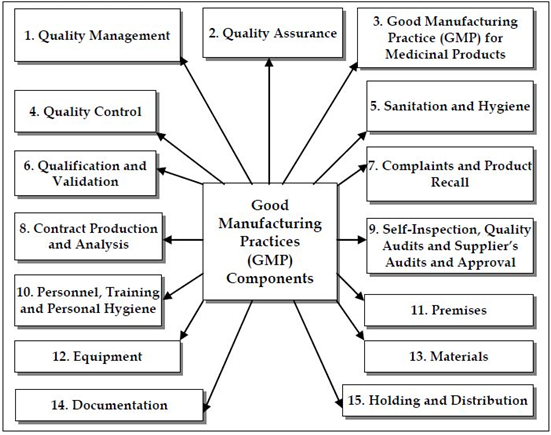
GMP GLP Road Map
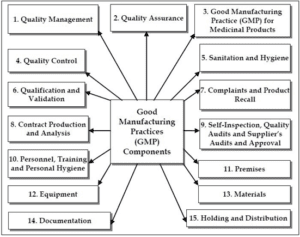

Core Compliance provides a clear road map for GMP & GLP with providing systems that assure proper design, monitoring, and control of manufacturing processes and facilities to meet FDA requirements. We create customized training courses designed specifically for your manufacturing organization, personnel, lead & internal auditors, and field service staff. Manufacturers must establish and follow quality systems to help ensure that their products consistently meet applicable requirements and specifications. The quality systems for FDA-regulated products (food, drugs, biologic, and devices) are known as current good manufacturing practices (CGMP’s). CGMP requirements for devices in part 820 (21 CFR part 820) were first authorized by section 520(f) of the Federal Food, Drug, and Cosmetic Act (the act).
GMP road map includes:
- Training of staff expectations best practices
- Requirements and objectives
- Develop & Implement Quality Management System, Quality Assurance Requirements- Personnel, Facility, Sanitation/Hygiene, Safety Self Inspection,Equipment, Handling/Packaging, Complaints & Product Recall, Supplier, Internal, & Lead Audits
- CFR record proposed rules, final rules of -FDA 21 Code of Federal Regulations Part 210 & 21 Code of Federal Regulations Part 211
- Instructional design
- Testing and/or evaluation of effectiveness GMP GLP Quote