Florida Regulatory Consulting Services
Florida Regulatory Consulting Services
 Core Compliance provides Florida regulatory consulting services to companies that require assistance to meet regulatory compliance. Our regulatory experts evaluate your current overall state of compliance, and provide a health check road map to ensure requirements are met for healthcare companies along manufacturers, suppliers and laboratories. We provide flexible international regulatory consulting services for all types of industries, medical devices, IVD pharmaceuticals, dietary supplements, biologics, no matter the stage.
Core Compliance provides Florida regulatory consulting services to companies that require assistance to meet regulatory compliance. Our regulatory experts evaluate your current overall state of compliance, and provide a health check road map to ensure requirements are met for healthcare companies along manufacturers, suppliers and laboratories. We provide flexible international regulatory consulting services for all types of industries, medical devices, IVD pharmaceuticals, dietary supplements, biologics, no matter the stage.
Florida Regulatory Consulting Services– can handle multiple aspects no matter the stage you’re products, pre-market review, product control, or post market surveillance. Core Compliance regulatory consultants can provide and ensure compliance and develop strategies that represent your organization and regulatory compliance. Our consultants not only provide guidance but also provide training to your medical device organization. Eliminate the carrying costs of long term high paid employees.
Ensure compliance with a regulatory expert liaison– between your company and the contractor with Global regulatory/FDA regarding product clearance. Coordinate, prepare, and follow-up with international regulations substantial change notifications- defend your submission during the review process with and assist with securing market clearance or approval.
FDA Regulatory Consultants in Florida
 Core Compliance provides FDA regulatory consultants in Florida at each stage to ensure compliance in ISO, FDA and international regulatory requirements. Our regulatory consultants assess overall current state, identify gaps to provide a health check that eliminates expensive costs of fines and delays to market, along with providing training for best practices to current employees. Utilize our regulatory expertise with flexibility to meet your needs below:
Core Compliance provides FDA regulatory consultants in Florida at each stage to ensure compliance in ISO, FDA and international regulatory requirements. Our regulatory consultants assess overall current state, identify gaps to provide a health check that eliminates expensive costs of fines and delays to market, along with providing training for best practices to current employees. Utilize our regulatory expertise with flexibility to meet your needs below:
- Hourly Ad Hoc
- Short-term project
- Long-term contractual agreement (Remote or Onsite)
Pre-market review, product control, or post market surveillance, our regulatory subject matter expertise can provide and ensure compliance.
- Education/Training-Changes and developments in the regulatory industry
- Road Map-Construct a strategy to and outline framework to pre-market review, product control, or post market surveillance
- Conception & Development
- Establish Process & Procedures, Controls
- Documentation requirements-Identify, Design, implement
- Drug/Product Control- Outline regulatory requirements
- Submission Management
- Manufacturing Facility- Inspection guidelines requirements
- Packaging/Complaint Handling- Identify Evaluate outline and create or change current procedures
- Software – Data management, Validation, Security
- Post-market surveillance-Outlined in the quality system requirements, internal auditing
Establish Process & Procedures, Controls
Core Compliance consultants establish processes, procedures and controls that meet your organization’s vision, goals and FDA, International regulatory compliance.
- Legal Requirements- Identify Global and US legal requirements
- Process the legalization and authentication of registration documents
- Identify, develop policy’s, processes, procedures, controls
- CAPA Management – establish a CAPA procedure early as part of an overall Quality Management System 21 CFR 820:100
- Compliant Procedures- 21 CFR 820.198
- Non-Conforming Product Procedures
- Vendor establishment control
ISO Quality Management System Requirements
 Core Compliance provides regulatory guidance to establish, design, and implement ISO quality management system requirements and quality assurance to the FDA. ISO 9001, ISO 13485, ISO 14971, FDA
Core Compliance provides regulatory guidance to establish, design, and implement ISO quality management system requirements and quality assurance to the FDA. ISO 9001, ISO 13485, ISO 14971, FDA
ISO FDA Requirements-
Products Requirements
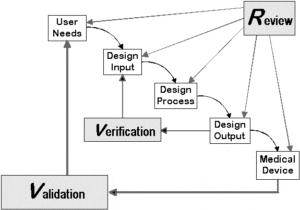 Our regulatory consultants provide guidance for products requirements, user needs, design input, design process, design output & validation.
Our regulatory consultants provide guidance for products requirements, user needs, design input, design process, design output & validation.
- Design Control Guidance, classification, product registration & listing-
- Unique Device Identifier (UDI). Identifying mark used to distinguish devices from one another and make them easier to track Device Identifier (Type of device) Production Identifier- (serial number, expiration date, date of manufacture)
- Proposed device changes
Regulatory Submission Management



We provide regulatory submission management including outlining requirements based upon medical device international regulatory requirements. Prepare and obtain certificates to foreign governments, Americas- United States, Brazil Canada Columbia Mexico, EMEA (Europe, the Middle East and Africa) Egypt, Europe CE Mark, Asia Pacific, Australia, New Zealand , Peru, Russia ,Saudi Arabia ,South Africa, Turkey, China, Hong Kong, India, Singapore, South Korea, Taiwan.
- Classification
- Registration/Approval
- Required Technical Documentation
- Conformity Assessment
- Declaration of conformity
Manufacturing Facilities
 Providing regulatory guidance for manufacturing facilities.
Providing regulatory guidance for manufacturing facilities.
- Inspection guidelines requirements- FDA Registration establishments or facilities) FDA requirements
- Non-Conforming devices form 483-(outlines and notifies management of any objectionable conditions found by FDA inspectors)
- CGMP– quality systems for FDA-regulated products (food, drugs, biologic, and devices) are known as current good manufacturing practices (CGMP’s)
Packaging Complaint Handling


Compliant handling is a vital part of compliance, we provide consulting that includes evaluating current procedures for packaging and labeling and create cGMP compliant regulatory requirements. Packaging and labeling to medical devices is a vital part of compliance to packaging systems in delivering clean, sterile and protected medical devices. External audit to identify packaging incidents or complaints.
Software Validation
 Core Compliance evaluates the current state of compliance for software validation and provides the following services:
Core Compliance evaluates the current state of compliance for software validation and provides the following services:
- Data management
- Validation Medical Device software-outline guidelines & recommendations
- Cyber-security and Risk Management processes.
Post-Market Surveillance
 Core Compliance can handle all of the post market surveillance needs including:
Core Compliance can handle all of the post market surveillance needs including:
- Support International Registrations for license renewals, product changes and/or new product launches
- Identify or Develop specific registration data information from applicable departments (R&D, Operations, QA, Medical,
- FDA/EU substantial change notifications
- Update technical files, as needed, with input from applicable departments
Represent Regulatory in product development projects by reviewing, approving, and completing requirements and coordinating team inputs for regulatory submissions. Compile and maintain regulatory documentation databases. Coordinate and respond to customer requests for product regulatory information.
ISO Standards
Core Compliance has expertise in the following ISO standards:
ISO 9001:2015 –Quality Management Systems
ISO 13485:2016 FDA Compliance 510K Submission CE Marking –Medical Devices Quality Management Systems
ISO 17025–Testing & Collaboration Laboratories Management
AS 9100–Aerospace Industry Quality Management Systems
ISO/IEC 20000:2011–Information Technology Service Management
ISO/IEC 27001:2011–Information Technology Security Management
R2:2013 RIOS Electronics Recycling NAID –Information Destruction e-Stewards –Responsible Recycling and Reuse of Electronics
ISO 14001– Environmental Management Systems
OHSAS 18001 ISO 45001 –Occupational Health & Safety Management
ISO/TS 16949-Automotive Industry Quality Management Systems
















